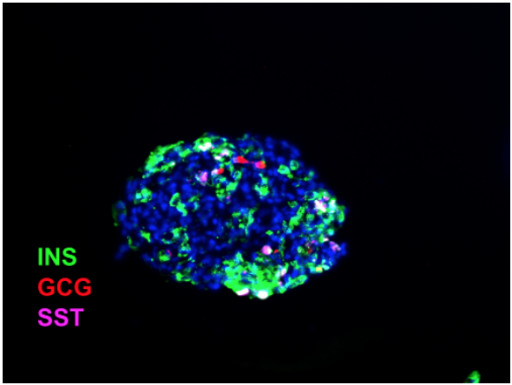
GERMANTOWN, Md., April 25, 2023 (Newswire.com) - Seraxis Inc., a pre-clinical stage company with a best-in-class islet replacement therapy for insulin-dependent diabetes, today announced the presentation of results from an efficacy study of Synthetic Replacement Endocrine (SRE) pancreatic clusters. These data were presented by William Rust, PhD, Chief Executive Officer at Seraxis, as an oral podium presentation at the 4th International Pancreas and Islet Transplant Association (IPITA) / Harvard Stem Cell Institute (HSCI) / Juvenile Diabetes Research Fund (JDRF) Summit, held from April 24-25, 2023 in Cambridge, Massachusetts. The presentation included an introduction to Seraxis' proprietary GMP process for manufacturing SRE clusters for nonclinical and clinical use.
Results from an ongoing type 1 diabetes efficacy study with SRE clusters (SR1423) implanted into the kidney capsule or gonadal fat pad in a diabetic mouse model showed that implantation resulted in sustained euglycemia. Control mice which did not receive an implant remained hyperglycemic. SRE clusters resemble human islets in morphology and distribution of the different hormones expressing cells.
"I am thrilled to report these strong efficacy results which demonstrate the superior performance of our lead product," said Dr. Rust. "These data combined with a safe profile which differentiates our stem cell derived therapy, are supportive of our path to the clinic in 2024."
Seraxis has developed a novel human multipotent immortal stem cell line, SR1423 that efficiently differentiates to clusters of functional endocrine pancreatic cells. These Synthetic Replacement Endocrine (SRE) clusters contain cell types that recapitulate native islet function in vitro and potently control blood glucose in vivo. Detailed characterization reveals significant distinctions between SRE, native islets and SC-beta cells. SRE clusters are manufactured following GMP requirements in closed vessels with large-scale formats and are being developed for near-term clinical studies.
IND filing and a Phase 1/2 clinical trial with SRE clusters are on track for 2023 and 2024 respectively.
ABOUT Seraxis Inc.
Seraxis' proprietary human stem cell line and manufacturing process efficiently generates SRE clusters that mimic native islets in purity and potency and have shown the potential to reverse diabetes in animal models. Seraxis has also developed the SeraGraft device and other tools to enable survival and function of the islet replacement therapy in immunocompetent hosts. Seraxis is a privately held biotechnology company with operations located in the BioHealth Capital Region, Maryland. Further information can be found at www.seraxis.com
Contact Information:Carole Welsch
Chief Business Officer
[email protected]
(240)4291347
Original Source: Seraxis Presents Successful Results Using Synthetic Replacement Endocrine Clusters for Type 1 Diabetes at the 4th IPITA / HSCI / JDRF Summit
The post Seraxis Presents Successful Results Using Synthetic Replacement Endocrine Clusters for Type 1 Diabetes at the 4th IPITA / HSCI / JDRF Summit first appeared on Enrose Magazine.
Medicine and Healthcare - Enrose Magazine originally published at Medicine and Healthcare - Enrose Magazine